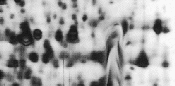
| Distortion of 2D Pattern |
| |
|
Vertical gel format
Uneven polymerization of gel due to incomplete polymerization, too rapid polymerization, or leakage during gel casting. |
Degas the gel solution.
Polymerization can be accelerated by increasing by 50% the amount of ammonium persulfate and TEMED used. Polymerization can be slowed by decreasing by 33% the amount of ammonium persulfate and TEMED used.
Ensure that there is no leakage during gel casting. |
| |
|
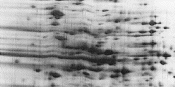
| Horizontal Streaking or Incompletely Focused Spots |
| |

|
Sample not completely solubilized prior to application.
|
Make sure that the sample is completely and stably solubilized. Repeated precipitation-resolubilization cycles produce or increase horizontal streaking.
|
| |
Sample is poorly soluble in rehydration solution. |
Increase the concentration of the solubilizing components in the rehydration solution.
Increase concentration of IPG buffer. |
| |
|
Interfering substrates. Nonprotein impurities in the sample can interfere with IEF, causing horizontal streaking in the final 2D result, particularly toward the acidic side of the gel. |
Modify sample preparation to limit these contaminants. |
| |

|
Ionic impurities in sample. |
Reduce salt concentration to below 10 mm by dilution or desalt the sample by dialysis. Precipitation with TCA and acetone and subsequent resuspension is another effective desalting technique that removes lipids, nucleotides, and other small molecules. Specific and nonspecific losses of proteins can occur with dialysis, gel chromatography, and precipitation/resuspension of samples.
If the sample cannot be modified, reduce the effect of ionic impurities by modifying the IEF protocol. Limit the voltage to 100–150 V for 2 hours, then resume a normal voltage step program. This pre-step allows the ions in the sample to move to the ends of the IPG strip. |
| |
| |
Ionic detergent in sample. |
If the ionic detergent SDS is used in sample preparation, the final concentration must not exceed 0.25% after dilution into the rehydration solution. Additionally, the concentration of the nonionic detergent present must be at least 8 times higher than the concentration of any ionic detergent to ensure complete removal of SDS from the proteins. |
| |

|
High sample load. |
Load less sample.
Micropreparative separations require clean sample. Modify sample preparation to limit concentrations.
Program a low initial voltage gradually. Extend focusing time. |
| |
|
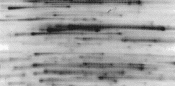
Underfocusing. Focusing time was not long enough to achieve steady-state focusing. |
Prolong focusing time. |
| |
|
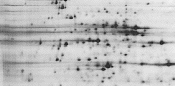
Overfocusing. Extended focusing times (>100,000 Vh) may result in electro-endosmotic water and protein movement, which can produce horizontal smearing. |
Reduce focusing time. |
| |
|
| Vertical Streaking |
| |
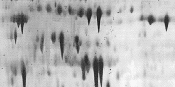
|
Insufficient equilibration.
Second-dimension buffer solutions prepared incorrectly.
Insufficient SDS in SDS electrophoresis buffer. |
Prolong equilibration time.
Prepare fresh solutions.
Use 0.1% (w/v) SDS. |
| |
|
| Vertical Gap in 2D Pattern |
| |
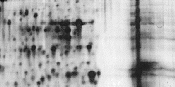
|
Impurities in sample. |
Modify sample preparation. |
| |

|
Impurities in rehydration solution components. |
Use only high-quality reagents.
Deionize urea solutions. |
| |
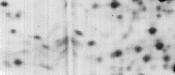
|
Bubble between IPG strip and top surface of second-dimension gel. |
Ensure that no bubbles are trapped between IPG strip and the top surface of the second-dimension gel. |
| |
|
| Vertical Regions of Poor focusing |
| |
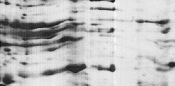
|
The IPG strip was not fully rehydrated. |
Ensure that the IPG strips are rehydrated with sufficient volume of rehydration solution.
Remove any large bubbles trapped under the IPG strip after rehydration solution is applied.
Check that the rehydration solution is evenly spread along the entire length of the IPG strip. |
| |
|
| Point Streaking |
| |
|
Silver staining
Dirty plates used to cast gel or particulate material on the surface of the gel. DTT and other thiol reducing agents exacerbate this effect. |
Properly wash glass plates. Scavenge any excess or residual thiol reducing agent with iodoacetamide before loading the IPG strips onto the second-dimension gel. |

