Appendices 1 and 2—References
Aitken A., Howell S., Jones D., Madrazo J., and Patel Y. 1995. 14-3-3  and and 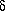 are the phosphorylated forms of Raf-activating 14-3-3 are the phosphorylated forms of Raf-activating 14-3-3 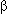 and and 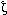 . In vivo stoichiometric phosphorylation in brain at a Ser-Pro-Glu-Lys motif. J. Biol. Chem. 270: 5706–5709. . In vivo stoichiometric phosphorylation in brain at a Ser-Pro-Glu-Lys motif. J. Biol. Chem. 270: 5706–5709.
Akins R.E. and Tuan R.S. 1992. Measurement of protein in 20 seconds using a microwave BCA assay. Biotechniques 12: 496–499.
Annan W.D., Manson W., and Nimmo J.A. 1982. The identification of phosphoseryl residues during the determination of amino acid sequence in phosphoproteins. Anal. Biochem. 121: 62–68.
Baker B.M. and Murphy K.P. 1996. Evaluation of linked protonation effects in protein binding using isothermal titration calorimetry. Biophys. J. 71: 2049–2055.
Baumann G., Frommel C., and Sander C. 1989. Polarity as a criterion in protein design. Protein Eng. 2: 329–334.
Biltonen R.L. and Langerman N. 1979. Microcalorimetry for biological chemistry: Experimental design, data analysis and interpretation. Methods Enzymol. 61: 287–319.
Blanchard J.S. 1984. Buffers for enzymes. Methods Enzymol. 104: 404–414.
Blume-Jensen P. and Hunter T. 2001. Two-dimensional phosphoamino acid analysis. Methods Mol. Biol. 124: 49–65.
Bollag D.M., Rozycki M.D., and Edelstein S.J. 1996. Protein methods, 2nd edition, pp. 76–78. Wiley, New York.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye ligand binding. Anal. Biochem. 72: 248–254.
Brown R.E., Jarvis K.L., and Hyland K.J. 1989. Protein measurement using bicinchoninic acid: Elimination of interfering substances. Anal. Biochem. 180: 136–139.
Burlingame A.L. and Carr S.A., eds. 1996. Mass spectrometry in the biological sciences. Humana Press, Totowa, New Jersey.
Cantor C.R. and Schimmel P.R. 1980. Biophysical chemistry: The behavior of biological macromolecules. W.H. Freeman, New York.
Dawson R.M.C., Elliott D.C., Elliott W.H., and Jones K.M., eds. 1969. Data for biochemical research. Clarendon Press, Oxford, United Kingdom.
Darby N.J. and Creighton T.E. 1993. Protein structure. In In focus (ed. D. Rickwood), p. 4. IRL Press, Oxford, United Kingdom.
Doyle M.L. and Hensley P. 1998. Tight ligand binding affinities determined from thermodynamic linkage to temperature by titration calorimetry. Methods Enzymol. 295: 88–99.
Doyle M.L., Louie G.L., Dal Monte P.R., and Sokoloski T.D. 1995. Tight binding affinities determined from linkage to protons by titration calorimetry. Methods Enzymol. 259: 183–194.
Fanger B.O. 1987. Adaptation of the Bradford Protein Assay to membrane-bound proteins by solubilizing in glucopyranoside detergents. Anal. Biochem. 162: 11–17.
Flogel M. and Biltonen R.L. 1975. Calorimetric and potentiometric characterization of the ionization behavior of ribonuclease A and its complex with 3'-cytosine monophosphate. Biochemistry 14: 2603–2609.
Freire E., Mayorga O.L., and Straume M. 1990. Isothermal titration calorimetry. Anal. Chem. 62: 950A–959A.
Friedenauer S. and Berlet H.H. 1989. Sensitivity and variability of the Bradford Protein Assay in the presence of detergents. Anal. Biochem. 178: 263–268.
Gomez J. and Freire E. 1995. Thermodynamic mapping of the inhibitor site of the aspartic protease endothiapepsin. J. Mol. Biol. 252: 337–350.
Grimes B.A., Meyers J.J., and Liapis A.I. 2000. Determination of the intraparticle electroosmotic volumetric flow-rate, velocity and Peclet number in capillary electrochromatography from pore network theory. J. Chromatogr. 890: 61–72.
Gygi S.P., Rist B., Gerber S.A., Turecek F., Gelb M.H., and Aebersold R. 1999. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17: 994–999.
Hagel P., Gerding J.J.T., Fieggen W., and Bloemendal H. 1971. Cyanate formation in solutions of urea. I. Calculations of cyanate concentrations at different temperature and pH. Biochem. Biophys. Acta 243: 366–373.
Harlow and Lane. 1999.
Hill H.D. and Straka J.G. 1988. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal. Biochem. 170: 203–208.
Hinson D.L. and Webber R.J. 1988. Miniaturization of the BCA protein assay. BioTechniques 6: 14–16.
Holmes C.F.B. 1987. A new method for the selective isolation of phosphoserine-containing peptides. FEBS Lett. 215: 21–24.
Indyk L. and Fisher H.F. 1998. Theoretical aspects of isothermal titration calorimetry. Methods Enzymol. 295: 350–364.
Isobe T., Takahashi N., and Putnam F.W. 1991a. Protein and peptide mapping by two-dimensional HPLC. In HPLC of peptides and proteins: Separation, analysis and conformation (ed. R.S. Hodges et al.), pp. 835–845. CRC Press, New York.
Isobe T., Uchida K., Taoka M., Shinkai F., Manabe T., and Okuyama T. 1991b. Automated high performance liquid chromatographic system for mapping proteins in highly complex mixtures. J. Chromatogr. 588: 115–123.
Janin J. 1979. Surface and inside volumes in globular proteins. Nature 277: 491–492.
Jelesarov I. and Bosshard H.R. 1999. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J. Mol. Recognit. 12: 3–18.
Kaushal V. and Barnes L.D. 1986. Effect of zwitterionic buffers on measurement of small masses of protein with bicinchoninic acid. Anal. Biochem. 157: 291–294.
Kessler R.J. and Fanestil D.D. 1986. Interference by lipids in the determination of protein using bicinchoninic acid. Anal. Biochem. 159: 138–142.
Krishna and Wold 1997. [Qau ( au. initial(s), article title journal, vol., and inclusive pp.]
Leavitt S. and Freire E. 2001. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 11: 560–566.
Lee B. and Richards F.M. 1971. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 55: 379–400.
Liapis A.I. and Grimes B.A. 2000. Modeling the velocity field of the electroosmotic flow in charged capillaries and in capillary columns packed with charged particles: Interstitial and intraparticle velocities in capillary electrochromatography systems. J. Chromatogr. 877: 181–215.
Link A.J., Eng J., Schieltz D.M., Carmack E., Mize G.J., Morris D.R., Garvik B.M., and Yates J.R. 1999. Direct analysis of protein complexes using mass spectrometry. Nat. Biotechnol. 17: 676–682.
Lovatt M., Cooper A., and Camilleri P. 1996. Energetics of cyclodextrin-induced dissociation of insulin oligomers. Eur. Biophys. J. 24: 354–357.
Luque I. and Freire E. 1998. A system for the structure-based prediction of binding affinities and molecular design of peptide ligands. Methods Enzymol. 295: 100–127.
Matyska M.T., Pesek J.J., Boysen R.I., and Hearn M.T.W. 2001. Characterization of open tubular capillary electrochromatography columns for the analysis of synthetic peptides using isocratic conditions. Anal. Chem. 73: 5116–5125.
Meyer H.E., Hoffmann-Posorske E., Korte H., and Heilmeyer L.M.G., Jr. 1986. Sequence analysis of phosphoserine-containing peptides. Modification for picomolar sensitivity. FEBS Lett. 204: 61–66.
Miller S., Janin J., Lesk A.M., and Chothia C. 1987. Interior and surface of monomeric proteins. J. Mol. Biol. 196: 641–656.
Parker M.H., Lunney E.A., Ortwine D.F., Pavlovsky A.G., Humblet C., and Brouillette C.G. 1999. Analysis of the binding of hydroxamic acid and carboxylic acid inhibitors to the stromelysin-1 (matrix metalloproteinase-3) catalytic domain by isothermal titration calorimetry. Biochemistry 38: 13592–13601.
Peterson G.L. 1983. Determination of total protein. Methods Enzymol. 91: 95–121.
Pierce J. and Suelter C.H. 1977. An evaluation of the Coomassie brilliant blue G-250 dye-binding method for quantitative protein determination. Anal. Biochem. 81: 478–480.
Radzicka A. and Wolfenden R. 1988. Comparing the polarities of the amino acids: Side-chain distribution coefficients between the vapor phase, cyclohexane, 1-octanol, and neutral aqueous solution. Biochemistry 27: 1664–1670.
Read S.M. and Northcote D.H. 1981. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 116: 53–64.
Richards F.M. 1977. Areas, volumes, packing and protein structure. Annu. Rev. Biophys. Bioeng. 6: 151–176.
Richardson W.S., Munksgaard E.C., and Butler W.T. 1978. Rat incisor phosphoprotein. The nature of the phosphate and quantitation of the phosphoserine. J. Biol. Chem. 253: 8042–8046.
Rose G.D., Geselowitz A.R., Lesser G.J., Lee R.H., and Zehfus M.H. 1985. Hydrophobicity of amino acids in globular proteins. Science 229: 834–838.
Ross G., Dittmann M., Bek F., and Rozing G. 1996. Capillary electrochromatography—Enhancement of LC separation in packed capillary columns by means of electrically driven mobile phases. Am. Lab. 28: 3–12.
Schien C.H. 1990. Solubility as a function of protein structure and solvent components. BioTechnology 8: 308–317.
Sedmak J.J. and Grossberg S.E. 1977. A rapid, sensitive and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 79: 544–552.
Sigurskjold B.W. 2000. Exact analysis of competition ligand binding by displacement isothermal titration calorimetry. Anal. Biochem. 277: 260–266.
Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Cocke N.M., Olson B.J., and Klenk D.C. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85.
Spector T. 1978. Refinement of the Coomassie Blue method of protein quantitation. Anal. Biochem. 86: 142–146.
Stoscheck C.M. 1990. Quantitation of protein. Methods Enzymol. 182: 50–68.
Straume M. and Freire E. 1992. Two-dimensional differential scanning calorimetry: Simultaneous resolution of intrinsic protein structural energetics and ligand binding interactions by global linkage analysis. Anal. Biochem. 203: 259–268.
Takahashi N., Isobe T., and Putnam F.W. 1991. Multidimensional, microscale HPLC technique in protein sequencing. In HPLC of proteins, peptides, and polynucleotides (ed. M.T.W. Hearn), pp. 307–330. VCH, New York.
Takahashi N., Ortel T.L., and Putnam F.W. 1984. Single-chain structure of human ceruloplasmin; the complete amino acid sequence of the whole molecule. Proc. Natl. Acad. Sci. 81: 390–394.
Takahashi N., Ishioka N., Takahashi Y., and Putnam F.W. 1985. Automated tandem high-performance liquid chromatographic system for separation of extremely complex peptide mixtures. J. Chromatogr. 326: 407–418.
Takahashi N., Takahashi Y., Blumberg B.S., and Putnam F.W. 1987a. Amino acid substitutions in genetic variants of human serum albumin and in sequences inferred from molecular cloning. Proc. Natl. Acad. Sci. 84: 4413–4417.
Takahashi N., Takahashi Y., Isobe T., Putnam F.W., Fujita M., Satoh C., and Neel J.V. 1987b. Amino acid substitutions in inherited albumin variants from Amerindian and Japanese populations. Proc. Natl. Acad. Sci. 84: 8001–8005.
Taoka M., Yamakuni T., Song S.Y., Yamakawa Y., Seta K., Okuyama T., and Isobe T. 1992. A rat cerebellar protein containing the cdc10/SW16 motif. Eur. J. Biochem. 207: 615–620.
Taoka M., Isobe T., Okuyama T., Watanabe M., Kondo H., Yamakawa Y., Ozawa F., Hishinuma F., Kubota M., Minegishi A., Song S.Y., and Yamakuni T. 1994. Murine cerebellar neurons express a novel gene encoding a protein related to cell cycle control and cell fate determination proteins. J. Biol. Chem. 269: 9946–9951.
Todd M.J., Luque I., Velazquez-Campoy A., and Freire E. 2000. Thermodynamic basis of resistance to HIV-1 protease inhibition: Calorimetric analysis of the V82F/I84V active site resistant mutant. Biochemistry 39: 11876–11883.
Trinquier G. and Sanejouand Y.-H. 1998. Which effective property of amino acids is best preserved by the genetic code? Protein Eng. 11: 153–169.
Van Holde K.E., Johnson W.C., and Ho P.S. 1998. Principles of physical biochemistry. Prentice Hall, Upper Saddle River, New Jersey.
Van Kley H. and Hale S.M. 1977. Assay for protein by dye binding. Anal. Biochem. 81: 485–487.
Velazquez-Campoy A., Kiso Y., and Freire E. 2001. The binding energetics of first- and second-generation HIV-1 protease inhibitors: Implications for drug design. Arch. Biochem. Biophys. 390: 169–175.
Velazquez-Campoy A., Todd M.J., and Freire E. 2000a. HIV-1 protease inhibitors: Enthalpic versus entropic optimization of the binding affinity. Biochemistry 39: 2201–2207.
_______. 2000b. Thermodynamic dissection of the binding energetics of KNI-272, a potent HIV-1 protease inhibitor. Protein Sci. 9: 1801–1809.
Walhagen K., Unger K.K., and Hearn M.T.W. 2000a. Capillary electroendoosmotic chromatography of peptides. J. Chromatogr. A 887: 165–185.
_______. 2000b. Influence of temperature on the behaviour of small linear peptides in capillary electrochromatography. J. Chromatogr. A. 893: 401–409.
Walhagen K., Unger K.K., and Hearn M.T.W. 2001. Capillary electrochromatography analysis of hormonal cyclic and linear peptides. Anal. Chem. 73: 4924–4936.
Wiechelman K.J., Braun R.B., and Fitzpatrick J.D. 1988. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal. Biochem. 175: 231–237.
Wilson C.M. 1979. Studies and critique of Amido black 10B, Coomassie blue R and Fast green FCF as stains for proteins after polyacrylamide gel electrophoresis. Anal. Biochem. 96: 263–278.
Wiseman T., Williston S., Brandts J.F., and Nin L.N. 1989. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179: 131–137.
Wyman J. and Gill S.J. 1990. Binding and linkage: Functional chemistry of biological macromolecules. University Science Books, Mill Valley, California.
Zhang S.H., Huang X., Zhang J., and Horvath C. 2000. Capillary electrochromatography of proteins and peptides with a cationic acrylic monolith. J. Chromatogr. 887: 465–477.
|

