Chapter 7: Peptide Mapping and Sequence Analysis of Gel–resolved Proteins—References
Aebersold R. 1993. Internal amino acid sequence of proteins after in situ protease digestion on nitrocellulose. In A practical guide for protein and peptide purification for microsequencing, 2nd edition (ed. P. Matsudaira), pp. 73–81. Academic Press, New York.
Aebersold R.H., Leavitt J., Saavedra R.A., Hood L.E., and Kent S.B. 1987. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc. Natl. Acad. Sci. 84: 6970–6974.
Ambler R.P. 1965. The behaviour of peptides formed by cyanogen bromide cleavage of proteins. Biochem. J. 96: 32.
Anderson W.L. and Wetlaufer D.B. 1975. A new method for disulfide analysis of peptides. Anal. Biochem. 67: 493–502.
Armentrout R.W. and Doolittle R.F. 1969. Pyrrolidonecarboxylyl peptidase: Stabilization and purification. Arch. Biochem. Biophys. 132: 80–90.
Atassi M.Z. and Habeeb A.F.S.A. 1972. Reaction of proteins with citraconic anhydride. Methods Enzymol. 25: 546–553.
Baumeister W., Walz J., Zühl F., and Seemüller E. 1998. The proteasome: Paradigm of a self-compartmentalizing protease. Cell 92: 367–380.
Bergman T. and Jörnvall H. 1987. Electroblotting of individual polypeptides from SDS/polyacrylamide gels for direct sequence analysis. Eur. J. Biochem. 169: 9–12.
Blumenfeld O.O., Rojkind M., and Gallop P.M. 1965. Subunits of hydroxylamine-treated tropocollagen. Biochemistry 4: 1780–1788.
Bond J.S. and Beynon R.J., eds. 1989. Proteolytic enzymes: A practical approach. IRL Press/Oxford University Press, Oxford, United Kingdom.
Bornstein P. and Balian G. 1977. Cleavage at Asn-Gly bonds with hydroxlyamine. Methods Enzymol. 47: 132–145.
Brown J.L. 1979. A comparison of the turnover of alpha-N-acetylated and nonacetylated mouse L-cell proteins. J. Biol. Chem. 254: 1447–1449.
Brown J.L. and Roberts W.K. 1976. Evidence that approximately 80 per cent of the soluble proteins from Ehrlich ascites cells are N -acetylated. J. Biol. Chem. 251: 1009–1014. -acetylated. J. Biol. Chem. 251: 1009–1014.
Brune D.C. 1992. Alkylation of cystein with acrylamide for protein sequence analysis. Anal. Biochem. 207: 285–290.
Burns J.A., Butler J.C., Moran J., and Whitesides G.M. 1991. Selective reduction of disulfides by tris(2-carboxyethyl)phosphine. J. Org. Chem. 56: 2648–2650.
Burnstein Y. and Patchornik A. 1972. Selective chemical cleavage of tryptophanyl peptide bonds in peptides and proteins. Biochemistry 11: 4641–4650.
Candiano G., Porotto M., Lanciotto M., and Ghiggeri G.M. 1996. Negative staining of proteins in polyacrylamide gels with methyl trichloroacetate. Anal. Biochem. 243: 245–248.
Casero P., Del Campo G.B., and Righetti P.G. 1985. Negative aurodye for polyacrylamide gels: The impossible stain. Electrophoresis 6: 362–372.
Castellanos-Serra L., Proenza W., Huerta V., Moritz R.L, and Simpson R.J. 1999. Proteome analysis of polyacrylamide gel-separated proteins visualized by reversible negative staining using imidazole-zinc salts. Electrophoresis 20: 732–737.
Cavins J.F. and Friedman M.A. 1970. An internal standard for amino acid analyses: S-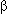 -(4-pyridylethyl)-l-cysteine. Anal. Biochem. 35: 489–493. -(4-pyridylethyl)-l-cysteine. Anal. Biochem. 35: 489–493.
Cecil R. 1963. Intramolecular bonds in proteins. The role of sulfur in proteins. In The proteins, 2nd edition (ed. H. Neurath), vol. 1, pp. 379–476. Academic Press, New York.
Cleland W.W. 1964. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 3: 480–482.
Cleveland D.W., Fischer S.G., Kirschner M.W., and Laemmli U.K. 1977. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 252: 1102–1106.
Cole A.R., Hall N.E., Eddes J.S., Reid G.E., Moritz R.L., Treutlein H., and Simpson R.J. 1999. Disulfide bond structure and N-glycosylation sites of the extracellular domain of the human interleukin-6 receptor. J. Biol. Chem. 274: 7207–7215.
Cole E.G. and Mecham D.K. 1966. Cyanate formation and electrophoretic behaviour of proteins in gels containing urea. Anal. Biochem. 14: 215–222.
Cole R.D. 1961. On the transformation of insulin in concentrated solutions of urea. J. Biol. Chem. 236: 2670–2671.
Crestfield A.M., Moore S., and Stein W.H. 1963. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J. Biol. Chem. 238: 622–627.
Dixon H.B.F. and Perham R.N. 1968. Reversible blocking of amino groups with citraconic anhydride. Biochem. J. 109: 312–314.
Drapeau G.R. 1977. Cleavage at glutanaic acid with staphylococcal protease. Methods Enzymol. 47: 189–191.
_______. 1978. The primary structure of staphylococcal protease. Can. J. Biochem. 56: 534–544.
_______. 1980. Substrate specificity of a proteolytic enzyme isolated from a mutant of Pseudomonas fragi. J. Biol. Chem. 255: 839–840.
Du H., Simpson R.J., Clarke A.E., and Bacic A. 1996. Molecular characterization of a stigma-specific gene encoding and arabinogalactan-protein (AGP) from Nicotiana alata. Plant J. 9: 313–323.
Eckerskorn C. and Lottspeich F. 1989. Internal amino acid sequence analysis of proteins separated by gel electrophoresis after tryptic digestion in polyacrylamide matrix. Chromatographia 28: 92–94.
Ellman G.L. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82: 70–77.
Fernandez J., DeMott M., Atherton D., and Mische S.M. 1992. Internal protein sequence analysis: Enzymatic digestion for less than 10 micrograms of protein bound to polyvinylidene fluoride or nitrocellulose membranes. Anal. Biochem. 201: 255–264.
Fernandez-Patron C., Hardy E., Sosa A., Seoane J., and Castellanon L. 1995a. Double staining of Coomassie blue-stained polyacrylamide gels by imidazole-sodium dodecyl sulfate-zinc reverse staining: Sensitive detection of Coomassie blue-undetected proteins. Anal. Biochem. 224: 263–269.
Fernandez-Patron C., Castellanos-Serra L., Hardy E., Guerra M., Estevez E., Mehl E. and Frank R.W. 1998. Understanding the mechanism of the zinc-ion stains of biomacromolecules in electrophoresis gels: Generalization of the reverse-staining technique. Electrophoresis 19: 2398–2406.
Fernandez-Patron C., Calero M., Collazo P.R., Garcia J.R., Madrazo J., Musacchio A., Soriano F., Estrada R., Frank R., Castellanos-Serra L.R., and Mendez E. 1995b. Protein reverse staining: High-efficiency microanalysis of unmodified proteins detected on electrophoresis gels. Anal. Biochem. 224: 203–211.
Fontana A. and Gross E. 1986. Fragmentation of polypeptides by chemical methods. In Practical protein chemistry (ed. A. Darbyre), pp. 67–120. Wiley, Chichester, United Kingdom.
_______. 1987. Fragmentation of polypeptides by chemical methods. In Practical protein chemistry: A handbook (ed. A. Darbre), pp. 67–120. Wiley, New York.
Fontana A., Dalzoppo D., Grandi C., and Zambonin M. 1983. Cleavage at tryptophan with  -iodosobenzoic acid. Methods Enzymol. 91: 311–318. -iodosobenzoic acid. Methods Enzymol. 91: 311–318.
Getz E.B., Xiao M., Chakrabarty T., Cooke R., and Selvin P.R. 1999. A comparison between sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal. Biochem. 273: 73–80.
Gevaert K. and Vandekerckhove J. 2000. Protein identification methods in proteomics. Electrophoresis 21: 1145–1154.
Gibbons I. and Perham R.N. 1970. The reaction of aldolase with 2-methylmaleic anhydride. Biochem. J. 116: 843–849.
Gray W.R. 1993. Disulfide structures of highly bridged peptides: A new strategy for analysis. Protein Sci. 2: 1732–1748.
Grego B., Van Driel I.R., Stearne P.A., Goding J.W., Nice E.C., and Simpson R.J. 1985. A microbore high performance liquid chromatography strategy for the purification of polypeptides for gas-phase sequence analysis: Structural studies on the murine trasferrine receptor. Eur. J. Biochem. 148: 485–491.
Gross E. and Witkop B. 1961. Selective cleavage of the methionyl peptide bonds in ribonuclease with cyanogen bromide. J. Am. Chem. Soc. 83: 1510–1511.
Habeeb A.F.S.A. 1972. Reaction of protein sulfhydryl groups with Ellman's reagent. Methods Enzymol. 25: 457–464.
Hagel P., Gerding J.J.T., Fieggen W., and Bloemendal H. 1971. Cyanate formation in solutions of urea. I. Calculation of cyanate concentrations at different temperature and pH. Biochem. Biophys. Acta 243: 366–373.
Hagmann M.-L. 1988. Handbook of proteolytic enzymes (ed. A.J. Barrett et al.), pp. 1542–1543. Academic Press, New York.
Hagmann M.-L., Geuss U., Fischer S., and Kresse G.-B. 1995. Peptidyl-Asp metalloendopeptidase. Methods Enzymol. 248: 782–787.
Harlow E. and Lane D., eds. 1999. Using antibodies: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Heinrickson R.L. 1977. Applications of thermolysin in protein structural analysis. Methods Enzymol. 47: 175–189.
Hellman U., Wernstedt C., Gonez J., and Heldin C.-H. 1995. Improvement of an "in-gel" digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 224: 451–455.
Henzel W.J., Billeci T.M., Stults J.T., Wong S.C., Grimley C., and Watanabe C. 1993. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc. Natl. Acad. Sci. 90: 5011–5015.
Herbert B. 1999. Advances in protein solubilisation for two-dimensional gel electrophoresis. Electrophoresis 20: 650–663.
Herbert B., Molloy M.P., Gooley A.A., Walsh B.J., Bryson W.G., and Williams K.L. 1998. Improved protein solubility in two-dimensional-gel electrophoresis using tributyl phosphine as a reducing agent. Electrophoresis 19: 845–851.
Hershko A. and Ciechanover A. 1998. The ubiquitin system. Annu. Rev. Biochem. 67: 425–480.
Higgins R.C. and Dahmus M.E. 1979. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal. Biochem. 93: 257–260.
Hirs C.H.W. 1967. Determination of cystine as cysteic acid. Methods Enzymol. 11: 59–62.
Horn M. and Laursen R.A. 1973. Solid-phase Edman degradation. Attachment of carboxy-terminal homoserine peptides to an insoluble resin. FEBS Lett. 36: 285–288.
Houmard J. and Drapeau G.P. 1972. Staphylococcal protease: A proteolytic enzyme specific for glutamoyl bonds. Proc. Natl. Acad. Sci. 69: 3506–3509.
Hunkapiller M.W., Luhan E., Ostrander F., and Hood L.E. 1983. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 91: 227–236.
Inglis A.S. and Edman P. 1970. Mechanism of cyanogen bromide reaction with methionine in peptides and proteins. Anal. Biochem. 37: 73–80.
Inglis A.S. and Liu T.-Y. 1970. The stability of cysteine and cystine during acid hydrolysis of proteins and peptides. J. Biol. Chem. 245: 112–116.
Iwamatsu A. 1992. S-carboxymethylation of proteins transferred onto polyvinylidene difluoride membranes followed by in situ protease digestion and amino acid microsequencing. Electrophoresis 13: 142–147.
Jacobson G.R., Schaffer M.H., Stark G.R., and Vanaman T.C. 1973. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J. Biol. Chem. 248: 6583–6591.
Jahnen W., Ward L.D., Reid G.E., Moritz R.L., and Simpson R.J. 1990. Internal amino acid sequencing of proteins by in situ cyanogen bromide cleavage in polyacrylamide gels. Biochem. Biophys. Res. Commun. 166: 139–145.
Jemmerson R. and Paterson Y. 1986. Mapping epitopes on a protein antigen by the proteolysis of antigen-antibody complexes. Science 232: 1001–1004.
Jenö P., Mini T., Moes S., Hintermann E., and Horst M. 1995. Internal sequences from proteins digested in polyacrylamide gels. Anal. Biochem. 224: 75–82.
Kasper C.B. 1975. Fragmentation of proteins for sequence studies and separation of peptide mixtures. In Protein sequence determination, 2nd edition. Mol. Biol. Biochem. Biophys. 8: 114–161.
Kawasaki H., Emori Y., and Suzuki K. 1990. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 191: 332–336.
Keil-Dlouhá V., Zylba N., Imhoff J.-M., Tong N.-T., and Keil B. 1971. Proteolytic activity of pseudotrypsin. FEBS Lett. 16: 291–295.
Kellner R. 1994. Chemical and enzymatic fragmentation of proteins. In Microcharacterization of proteins (ed. R. Kellner et al.), pp. 11–27. VCH, New York.
Kellner, R., Lottspeich F., and Meyer H.E., eds. 1994. Microcharacterization of proteins. VCH, New York.
Klotz I.M. 1967. Succinylation. Methods Enzymol. 11: 576–580.
Kobayashi K. and Smith J.A. 1987. Acyl-peptide hydrolase from rat liver. Characterization of enzyme reaction. J. Biol. Chem. 262: 11435–11445.
Konigsberg W. 1972. Reduction of disulfide bonds in proteins with dithiothreitol. Methods Enzymol. 25: 185–188.
Kwong M.Y. and Harris R.J. 1994. Identification of succinimide sites in proteins by N-terminal sequence analysis after alkaline hydroxylamine cleavage. Protein Sci. 3: 147–149.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–696.
Lee C., Levin A., and Branton D. 1987. Copper staining: A five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 166: 308–312.
Liu T.-Y. 1977. The role of sulfur in proteins. In The proteins, 3rd edition (ed. H. Neurath and R.L. Hill.), pp. 240–403. Academic Press, New York.
Lonnie D.A. and Weaver K.M. 1990. Detection and recovery of proteins from gels following zinc chloride staining. Appl. Theor. Electrophoresis 1: 279–282.
Lorand L., ed. 1981. Proteolytic enzymes. Methods Enzymol., vol. 80.
Lui M., Tempst P., and Erdjument-Bromage H. 1996. Methodical analysis of protein-nitrocellulose interactions to design a refined digestion protocol. Anal. Biochem. 241: 156–166.
Maclaren J.A. and Sweetman B.J. 1966. The preparation of reduced and S-alkylated wool keratins using TRI-n-butylphosphine. Aust. J. Chem. 19: 2355–2360.
Mahoney W.C. and Hermodson M.A. 1979. High yield cleavage of tryptophanyl bonds with  -iodosobenzoic acid. Biochemistry 18: 3810–3814. -iodosobenzoic acid. Biochemistry 18: 3810–3814.
Mahoney W.C., Smith P.K., and Hermodson M.A. 1981. Fragmentation of proteins with  -iodosobenzoic acid: Chemical mechanisms and identification of -iodosobenzoic acid: Chemical mechanisms and identification of  -iodosobenzoic acid as a reactive contaminant that modifies tyrosyl residues. Biochemistry 20: 443–448. -iodosobenzoic acid as a reactive contaminant that modifies tyrosyl residues. Biochemistry 20: 443–448.
Mant C.T. and Hodges R.S., eds. 1991. High-performance liquid chromatography of peptides and proteins: Separation, analysis, and conformation. CRC Press, Boca Raton, Florida.
Majors R.E. and Przybyciel M. 2002. Columns for reversed-phase LC separations in highly aqueous mobile phases. LCGC 20: 584–593 (www.chromatographyonline.com).
McCormack A.L., Schieltz D.M., Goode B., Yang S., Barnes G., Drubin D., and Yates J.R. 1997. Direct analysis and identification of proteins in mixtures by LC/MS/MS and database searching at the low-femtomole level. Anal. Biochem. 69: 767–776.
Merril C.R. 1987. Detection of proteins separated by electrophoresis. Adv. Electrophoresis 1: 111–139.
Merril C.R. and Washart K.M. 1998. Protein detection methods. In Gel electrophoresis of proteins. A practical approach, 3rd edition (ed. B.D. Hames), pp. 53–92. Oxford University Press, Oxford, United Kingdom.
Meyer T.S. and Lambert B.L. 1965. Use of Coomassie brilliant blue R250 for the electrophoresis of microgram quantities of parotid saliva proteins on acrylamide-gel strips. Biochem. Biophys. Acta 107: 144– 145.
Mitta M., Asada K., Uchimura Y., Kimizuka F., Kato I., Sakiyama F., and Tsunasawa S. 1989. The primary structure of porcine liver acylamino acid-releasing enzyme deduced from cDNA sequences. J. Biochem. 106: 548–551.
Moritz R.L. and Simpson R.J. 1992a. Application of capillary reversed-phase high-performance liquid chromatography to high-sensitivity protein sequence analysis. J. Chromatogr. 599: 119–130.
_______. 1992b. Purification of proteins and peptides for sequence analysis using microcolumn liquid chromatography. J. Microcol. Sep. 4: 485–489.
_______. 1993. Capillary liquid chromatography: A tool for protein structural analysis. In Methods in protein sequence analysis (ed. K. Imahori and F. Sakiyama), pp. 3–10. Plenum Press, New York.
Moritz R.L., Eddes J.S., Reid G.E., and Simpson R.J. 1996. S-pyridylethylation of intact polyacrylamide gels and in situ digestion of electrophoretically-separated proteins: A rapid mass spectrometric method for identifying cysteine-containing peptides. Electrophoresis 17: 907–917.
Moritz R.L., Hall N.E., Connolly L.M., and Simpson R.J. 2001. Determination of the disulfide structure and N-glycosylation sites of the extracellular domain of the human signal transducer gp130. J. Biol. Chem. 276: 8244–8253.
Moritz R.L., Reid G.E., Ward L.D., and Simpson R.J. 1994. Capillary HPLC: A method for protein isolation and peptide mapping. Methods 6: 213–226.
Moritz R.L., Eddes J.S., Ji H., Reid G.E., and Simpson R.J. 1995. Rapid separation of proteins and peptides using conventional silica-based supports: Identification of 2-D gel proteins following in-gel proteolysis. In Techniques in protein chemistry VI (ed. J.W. Crabb), pp. 311–319. Academic Press, San Diego, California.
Muzio M., Chinnaiyan A.M., Kischkel F.C., O'Rourke K., Shevchenko A., Ni J., Scaffidi C., Bretz J.D., Zhang M., Gentz R., Mann M., Krammer P.H., Peter M.E., and Dixit V.M. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85: 817–827.
Nelles L.P. and Bamburg J.R. 1976. Rapid visualization of protein-dodecyl-sulfate complexes in polyacrylamide gels. Anal. Biochem. 73: 522–531.
Neuhof V., Arold N., Taube D., and Ehrhardt W. 1988. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9: 255–262.
Omenn G.S., Fontana A., and Anfinsen C.B. 1970. Modification of the single tryptophan residue of staphylococcal nuclease by a new mild oxidizing agent. J. Biol. Chem. 245: 1895–1902.
Ortiz M.L., Calero M., Fernandez-Patron C., Patron C.F., Castellanos L., and Mendez E. 1992. Imidazole-SDS-Zn reverse staining of proteins in gels containing or not SDS and microsequence of individual unmodified electroblotted proteins. FEBS Lett. 296: 300–304.
Ozols J. and Gerard C. 1977. Covalent structure of the membranous segment of horse cytochrome b5: Chemical cleavage of the native hemoprotein. J. Biol. Chem. 252: 8549–8553.
Parham P., Matthew J., Androlewicz M.J., Brodsky F.M., Holmes N.J., and Ways J.P. 1982. Monoclonal antibodies: Purification, fragmentation, and application to structural and functional studies of class I MHC antigen. J. Immunol. Methods 53: 133–173.
Patterson S.D. and Aebersold R. 1995. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis 16: 1791–1814.
Patterson S.D., Hess D., Yungwirth T., and Aebersold R. 1992. High-yield recovery of electroblotted proteins and cleavage fragments from a cationic polyvinylidene fluoride-based membrane. Anal. Biochem. 202: 193–203.
Patton W.F., Lam L., Su Q., Lui M., Erdjument-Bromage H., and Tempst P. 1994. Metal chelates as reversible stains for detection of electroblotted proteins: Application to protein microsequencing and immunoblotting. Anal. Biochem. 220: 324–335.
Polverino de Laureto P., Scarmella E., Frigo M., Wondrich F.G., De Filippis V., Zambonin M., and Fontana A. 1999. Limited proteolysis of bovine  -lactalbumin: Isolation and characterization of protein domains. Protein Sci. 8: 2290–2303. -lactalbumin: Isolation and characterization of protein domains. Protein Sci. 8: 2290–2303.
Rabilloud T. 1990. Mechanisms of protein silver staining in polyacrylamide gels: A 10-year synthesis. Electrophoresis 11: 785–794.
_______. 1999. Silver staining of 2-D electrophoresis gels. Methods Mol. Biol. 112: 297–305.
_______. 2000. Detecting proteins separated by 2-D gel electrophoresis. Anal. Chem. 72: 48A–55A.
_______. 2001. A new silver staining apparatus and procedure for matrix/ionization-time of flight analysis of proteins after two-dimensional electrophoresis. Proteomics 1: 835–840.
Rabilloud T., Vuillard L., Gilly C., and Lawrence J.-J. 1994. Silver-staining of proteins in polyacrylamide gels: A general overview. Cell. Mol. Biol. 40: 57–75.
Raftery M.A. and Cole R.D. 1966. On the aminoethylation of proteins. J. Biol Chem. 241: 3457–3461.
Rawlings N.D. and Barrett A.J. 1993. Evolutionary families of peptidases. Biochem. J. 290: 205–218.
Rawlings N.D., O'Brien E., and Barrett A.J. 2002. MEROPS: The protease database. Nucleic Acids Res. 30: 343–346.
Renlund S., Klintrot I.-M., Nunn M., Schrimsher J.L., Wernstedt C., and Hellmann U. 1990. Peptide mapping of HIV polypeptides expressed in Escherichia coli—Quality control of different batches and identification of tryptic fragments containing residues of aromatic amino acids or cysteine. J. Chromatogr. 512: 325–335.
Riddles P.W., Blakeley R.L., and Zerner B. 1983. Reassessment of Ellman's reagent. Methods Enzymol. 91: 49–60.
Rittenhouse J. and Marcus F. 1984. Peptide mapping by polyacrylamide gel electrophoresis after cleavage at aspartyl-prolyl peptide bonds in sodium dodecyl sulfate-containing buffers. Anal. Biochem. 138: 442–448.
Riviere L.R., Fleming M., Elicone C., and Tempst P. 1991. Study and applications of the effects of detergents and chaotropes on enzymatic proteolysis. In Techniques in protein chemistry II (ed. J.J. Villafranca), pp. 171–179. Academic Press, San Diego, California.
Rosenfeld J., Capdevielle J., Guillemot J., and Ferrara P. 1992. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203: 173–179.
Rüegg U.T. and Rudinger J. 1977. Reductive cleavage of cystine disulfides with tributylphosphine. Methods Enzymol. 47: 111–116.
Sanger F. 1949. Fractionation of oxidized insulin. Biochem. J. 44: 126–128.
Saris C.J.M., van Eenbergen J., Jenks B.G., and Bloemers H.P.J. 1983. Hydroxylamine cleavage of proteins in polacrylamide gels. Anal. Biochem. 132: 54–67.
Savige W.E. and Fontana A. 1977. Cleavage of the tryptophanyl peptide bond by dimethyl sulfoxide-hydrobromic acid. Methods Enzymol. 47: 459–469.
Schaffner W. and Weissman C. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56: 502–514.
Schägger H. and von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range 1 to100 kDa. Anal. Biochem. 166: 368–397.
Schechter I. and Berger A. 1967. On the size of the active site in proteases. Biochem. Biophys. Res. Commun. 27: 157–162.
Shechter Y., Patchornik A., and Burstein Y. 1976. Selective chemical cleavage of tryptophanyl peptide bonds by oxidative chlorination with N-chlorosuccinimide. Biochemistry 15: 5071–5075.
Shevchenko A., Wilm M., Vorm O., and Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68: 850–858.
Simpson R.J. and Nice E.C. 1984. In situ cyanogen bromide cleavage of N-terminally blocked proteins in a gas-phase sequencer. Biochem. Int. 8: 787–791.
Simpson R. and Reid G.E. 1998. Sequence analysis of gel-resolved proteins. In Gel electrophoresis of proteins: A practical approach, 3rd edition (ed. B.D. Hames), pp. 237–267. Oxford University Press, Oxford, United Kingdom.
Simpson R.J., Neuberger M.R., and Liu T.-Y. 1976. Complete amino acid analysis of proteins from a single protein hydrolysate. J. Biol. Chem. 251: 1936–1940.
Simpson R.J., Moritz R.L., Nice E.C., and Grego B. 1987. A high-performance liquid chromatography procedure for recovering subnanomole amounts of protein from SDS-gel electroeluates for gas-phase sequence analysis. Eur. J. Biochem. 165: 21–29.
Simpson R.J., Moritz R.L., Rubira M.R., and Van Snick J. 1988a. Murine hybridoma/plasmacytoma growth factor. Eur. J. Biochem. 176: 187–197.
Simpson R.J., Moritz R.L., Van Roost E., and Van Snick J. 1988b. Characterization of a recombinant murine interleukin-6: Assignment of disulphide bonds. Biochem. Biophys. Res. Commun. 157: 364–372.
Simpson R.J., Moritz R.L., Begg G.S., Rubira M.R., and Nice E.C. 1989. Micropreparative procedures for high sensitivity sequencing of peptides and proteins. Anal. Biochem. 177: 221–236.
Snyder L.R. and Kirkland J.J. 1979. Introduction to modern liquid chromatography, 2nd edition. Wiley, New York.
Stark G.R., Stein W.H., and Moore S. 1960. Reactions of the cyanate present in aqueous urea with amino acids and proteins. J. Biol. Chem. 235: 3177–3181.
Stephenson R.C. and Clarke S. 1989. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J. Biol. Chem. 264: 6164–6170.
Stone K.L., McNulty D.E., LoPresti M.L., Crawford J.M., DeAngelis R., and Williams K.R. 1992. Elution and internal amino acid sequencing of PVDF-blotted proteins. In Techniques in protein chemistry III (ed. R. Angeletti), pp. 23–34. Academic Press, San Diego, California.
Sweetman B.J. and Maclaren J.A. 1966. The reduction of wool keratin by tertiary phosphines. Aust. J. Chem. 19: 2347–2354.
Switzer III R.C., Merril C.R., and Shifrin S. 1979. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal. Biochem. 98: 231–237.
Syrovy I. and Hodny Z. 1991. Staining and quantification of proteins separated by polyacrylamide gel electrophoresis. J. Chromatogr. 569: 175–196.
Titani K., Hermodson M.A., Ericsson L.H., Walsh K.A., and Neurath H. 1972. Amino acid sequence of thermolysin. Isolation and characterization of the fragments obtained by cleavage with cyanogen bromide. Biochemistry 11: 2427–2435.
Tong D., Moritz R.L., Eddes J.S., Reid G.E., Rasmussen R.K., Dorow D.S., and Simpson R.J. 1997. Fabrication of stable packed capillary reversed-phase columns for protein structural analysis. J. Prot. Chem. 16: 425–431.
Traub W. and Piez K.A. 1971. The chemistry and structure of collagen. Adv. Protein Chem. 25: 243–352.
Vorm O., Roepstorff P., and Mann M. 1994. Improved resolution and very high sensitivity in MALDI TOF of matrix surface made by fast evaporation. Anal. Chem. 66: 3281–3287.
Ward L.D., Reid G.E., Moritz R.L., and Simpson R.J. 1990. Strategies for internal amino acid sequence analysis of proteins separated by polyacrylamide gel electrophoresis. J. Chromatogr. 519: 199–216.
Welinder K.G. 1988. Generation of peptides suitable for sequence analysis by proteolytic cleavage in reversed-phase high-performance liquid chromatography solvents. Anal. Biochem. 174: 54–64.
Williams K., Kobayashi R., Lane W., and Tempst P. 1993. Internal amino acid sequencing: Observations from four different laboratories. Assoc. Biomol. Res. Fac. News 4: 7.
Wilson C.M. 1983. Staining of proteins on gels: Comparisons of dyes and procedures. Methods Enzymol. 91: 236–247.
Wu J. and Watson J.T. 1997. A novel methodology for assignment of disulfide bond pairings in proteins. Protein Sci. 6: 391–398.
Further Reading
Bond J.S. and Beynon R.J., eds. 1989. Proteolytic enzymes: A practical approach. IRL Press/Oxford University Press, Oxford, United Kingdom.
Fontana A. and Gross E. 1986. Fragmentation of polypeptides by chemical methods. In Practical protein chemistry (ed. A. Darbyre), pp. 67–120. Wiley, Chichester, United Kingdom.
Liu T.-Y. 1977. The role of sulfur in proteins. In The proteins, 3rd edition (ed. H. Neurath and R.L. Hill.), pp. 240–403. Academic Press, New York.
Lorand L., ed. 1981. Proteolytic enzymes. Methods Enzymol., vol. 80.
Snyder L.R. and Kirkland J.J. 1979. Introduction to modern liquid chromatography, 2nd edition. Wiley, New York.
|

