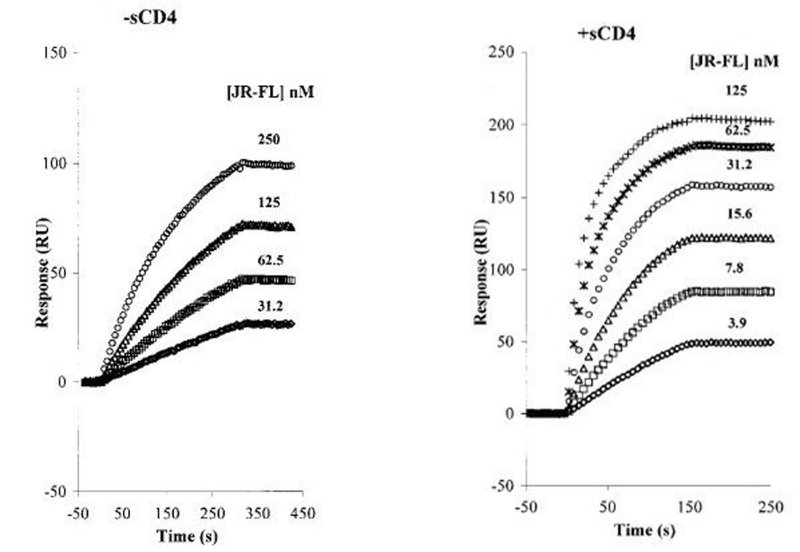

Figure 3. Sensorgram overlays for gp120 JR-FL binding to the immobilized mAb 17b surface. JR-FL gp120 protein concentrations are shown at the right of each sensorgram. (A) In the absence of sCD4. Flow rate is 30 μl min–1. (B) In the presence of sCD4; the sCD4 concentration in each mixture is 15 times that of each JR-FL concentration. Flow rate is 60 μl min–1. Global analysis of the reaction kinetics of the JRFL-17b interaction in the presence or absence of soluble CD4 (sCD4) using model fitting in the BIAevaluation software (BIAcore, AB) gives the following rates: JRFL-17b interaction, kon = 2.7 × 104 m –1 s–1, koff = 0.76 × 10–4 s–1, KD = 2.8 nm. (JRFL/CD4)-17b interaction, kon = 59 × 104 m –1 s–1, koff = 0.76 × 10-4 s-1, KD = 0.1 n m. Therefore, addition of sCD4 to the gp120-17b interaction results in a more than 20-fold increase in the affinity of gp120 for this antibody, mainly manifested in the association rate. This increase in affinity is attributed to a conformational rearrangement that occurs in gp120 upon binding of sCD4, which exposes the coreceptor site. The epitope for 17b overlaps with this site, which becomes still more exposed in the presence of sCD4. (Reprinted, with permission, from Zhang et al. 1999.)