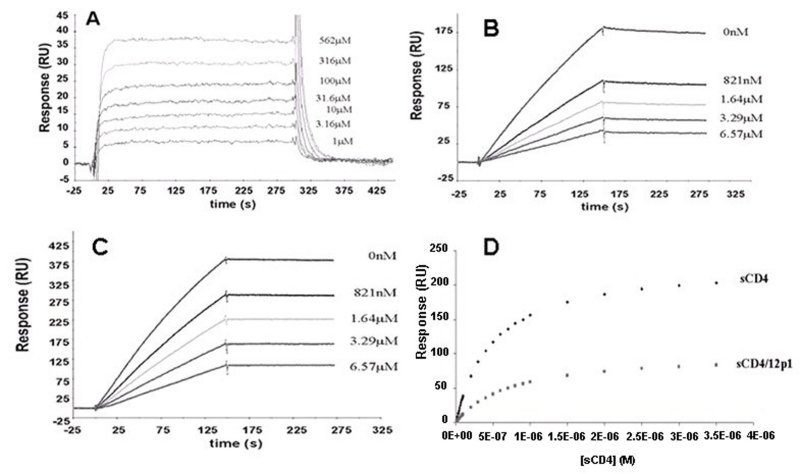

Figure 5. (A) Response curves for increasing 12p1 concentrations binding to immobilized HIV-1YU2 gp120. (B) 12p1 inhibition of gp120-sCD4 interaction. (C) 12p1 inhibition of gp120-17b interaction. (D) Analysis of sCD4 saturation of HIV-1YU2 gp120 in the presence versus absence of 12p1. Response at equilibrium (Req) values were obtained from fitting sensorgrams charting the response of immobilized HIV-1YU2 gp120 to increasing concentrations of sCD4 (0–3.5 μm) in the presence or absence of 100 μm 12p1. The resultant Req responses were then plotted versus sCD4 concentration and fitted to a steady-state 1:1 binding model to obtain the equilibrium binding constants KA and KD. Under the conditions used, the presence of 12p1 had no discernible effect on the kinetics of interaction between HIV-1YU2 gp120 and sCD4 (KD values, 721 nm and 429 nm, in the presence or absence of peptide, respectively) but had a marked effect on the total amount of sCD4 bound by HIV-1YU2 gp120. This indicates a noncompetitive mode of action for the 12p1 inhibitor. (Adapted from Biorn et al. 2004.)