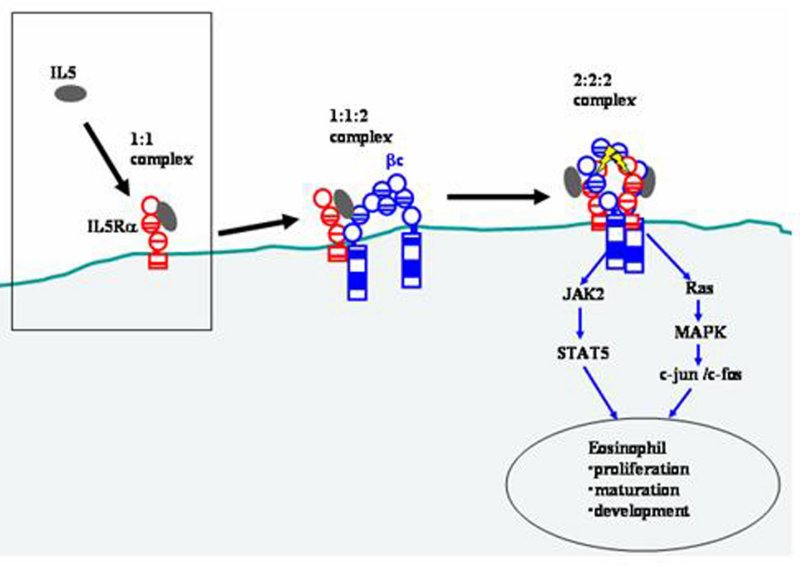

Figure 7. Proposed model of receptor assembly and activation by IL5. IL5 initially binds to IL5Rα to form a high-affinity 1:1 complex. Although IL5 is a homodimeric protein, it has been shown that the stoichiometry of IL5-IL5Rα interaction is 1:1 (Johanson et al. 1995). This IL5-IL5Rα complex is thought to bind to a preformed dimer of common receptor β (βc) to form 1:1:2 intermediate complex (McClure 2003). Subsequently, another IL5-IL5Rα complex binds to the 1:1:2 complex to form a 2:2:2 complex. It is proposed that this complex is subsequently disulfide-bonded between IL5Rα and βc and that it is this final complex which induces cytoplasmic sigaling through the JAK/STAT and MAPK pathways in eosinophils (Martinez-Moczygemba and Huston 2003). Protocol 1.4 addresses the initial chimokine-receptor interaction (shown in the box).