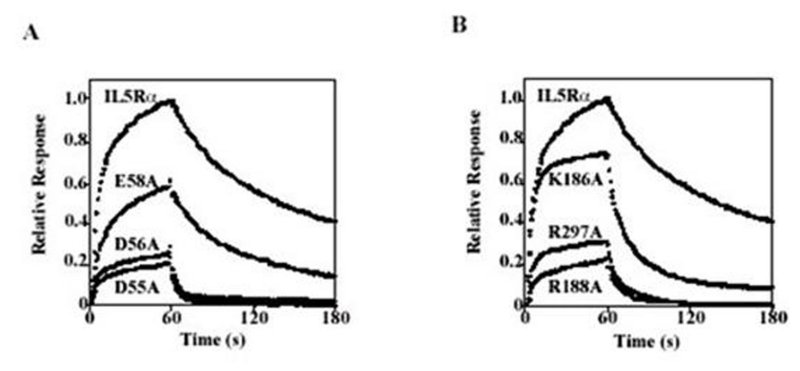

Figure 9. Mutations that diminish the high-affinity interaction of IL5Rα with IL5. Binding curves of the mutants D55A, D56A, and E58A (A) and the mutants K186A, R188A, and R297A (B), which markedly decrease the binding affinity. Each sample is injected onto the sensorchip at 0 seconds and is switched to buffer at 60 seconds. Each sensorgram shows the association (0–60 sec) and dissociation (60–180 sec) of IL5 (50 nm) to IL5Rαor the mutational variants captured by anti-V5 tag antibody. For comparison, sensorgrams are normalized depending on the amount of captured protein. Human IL5Rα comprises three fibronectin type III domains (D1, D2, and D3) in the extracellular region. Modeling of the tertiary structure of IL5Rα indicated that these binding residues fell into two clusters. The first cluster consists of D1 domain residues (D55A, D56A, and E58A) that form a negatively charged patch, whereas the second cluster consists of residues (K186A, R188A, and R297A) that form a positively charged patch at the interface of D2 and D3 domains. These results suggest that the IL5-IL5Rα system adopts a unique binding topology, in which the cytokine is recognized by a D2D3 tandem domain combined with a D1 domain, to form an extended cytokine recognition interface. (Adapted from Ishino et al. 2004.)